The https:// makes sure that you're connecting into the official Web page Which any information you offer is encrypted and transmitted securely.
Documents of manufacture (like distribution) that allow the whole historical past of the batch to become traced must be retained in the comprehensible and accessible form.
(b) Major products shall be discovered by a particular identification number or code that shall be recorded within the batch manufacturing document to indicate the particular equipment Utilized in the manufacture of each and every batch of a drug product or service.
Using IRIS for GMP inspections improves performance by harmonising and automating procedures and re-applying grasp knowledge held by EMA. In addition it simplifies retrieving and reporting details.
Given that cGMP utilizes the latest technological know-how to adhere to new manufacturing techniques, cGMP has a tendency to be costlier than GMP. Items that abide by cGMP also undergo much more testing to be sure the appropriate use in the created items and to guarantee the quality of pharmaceuticals. All the additional screening and innovative technological innovation make cGMP a far more pricey solution than GMP.
signifies anyone or organizational ingredient selected by the company to become liable for the responsibilities regarding high-quality control.
(b) There shall be created methods assigning responsibility for sanitation and describing in ample depth the cleansing schedules, techniques, gear, and products for use in cleaning the properties and facilities; such prepared strategies shall be followed.
The time period also features a completed dosage form that doesn't consist of an Energetic component but is intended to be used as a placebo.
. We see no value check here in the retention of this kind of labels Together with the needed gear log or batch history documentation. The labels provide a useful, momentary reason of positively figuring out The existing status of apparatus and the fabric beneath course of action.
A firm's justification for the frequency of media fills in relation to shifts need to be possibility primarily based, dependant upon the style of operations along with the media fill review structure. For shut
Should you’re manufacturing food items, drinks, or medication, you know you've got a sea of regulations and expectations which you need to hit and adhere to.
(one) There shall certainly be a written assessment of stability primarily based at the very least on screening or evaluation in the drug merchandise for compatibility on the elements, and based upon marketing encounter Using the drug product to indicate that there's no degradation with the merchandise for the traditional or predicted period of use.
We leverage reducing-edge read more enhancement and industrial manufacturing options to supply contract advancement & manufacturing companies from compact to large scale for oral solids, sterile injectables, smaller molecules and biologics; together with regulatory companies.
(a) All compounding and storage containers, processing strains, and big tools applied through the creation of a batch of the drug merchandise shall be adequately recognized all of the time to indicate their contents and, when needed, the stage of processing in the batch.
 Danny Tamberelli Then & Now!
Danny Tamberelli Then & Now! Haley Joel Osment Then & Now!
Haley Joel Osment Then & Now! Judge Reinhold Then & Now!
Judge Reinhold Then & Now!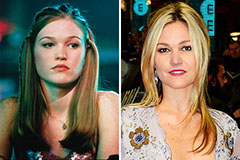 Julia Stiles Then & Now!
Julia Stiles Then & Now! Mason Reese Then & Now!
Mason Reese Then & Now!